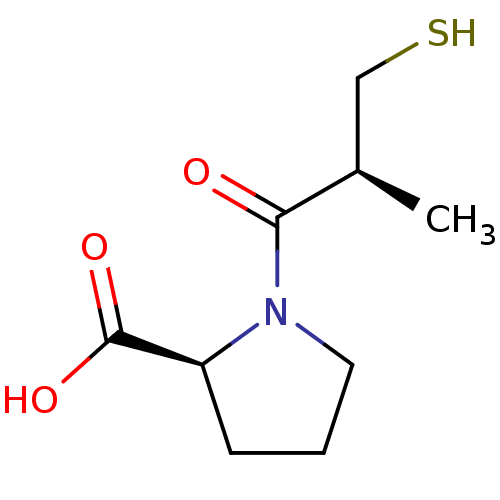
BDBM21642 (2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolidine-2-carboxylic acid::CHEMBL1560::Capozide::Captopril::Lopirin::SQ 14,225::SQ14534::US11491146, Compound L-Captopril
SMILES C[C@H](CS)C(=O)N1CCC[C@H]1C(O)=O
InChI Key InChIKey=FAKRSMQSSFJEIM-RQJHMYQMSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 52 hits for monomerid = 21642
Found 52 hits for monomerid = 21642
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataKi: 1.70nMAssay Description:Inhibitory activity against rabbit lung angiotensin-1 converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Inhibition of ACE (unknown origin) assessed as 3-Hydroxybutyril-glycil-glycil-glycine conversion to 3-hydroxybutyric acid after 60 mins by WST assayMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Specific activity of ACE was assayed in black 96-well microplates with or without various concentrations of inhibitors. N-Cbz-Phe-His-Leu was added, ...More data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataIC50: 2.30nMAssay Description:In vitro inhibitory activity was evaluated against angiotensin converting enzyme from rabbit in bovine buffered base. (reported from ref. 1b)More data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Compound was tested for its inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibitory concentration against angiotensin converting enzyme (ACE)More data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:In vitro inhibition of Angiotensin I converting enzymeMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:In vitro inhibition of angiotensin I converting enzyme (ACE)More data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataIC50: 22nMAssay Description:In vitro 50% inhibition of Angiotensin I converting enzymeMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Ability to inhibit Angiotensin I converting enzyme was determinedMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3More data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:In vitro inhibition of Angiotensin I converting enzyme in rabbit lungMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Inhibition of human ACE-mediated amyloid beta hydrolysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Inhibition of ACE (unknown origin) using 3-Hydroxybutylyl-Gly-Gly-Gly substrate assessed as reduction in 3-Hyroxybutylic acid generation incubated fo...More data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Inhibition of ACE (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Compound was tested for inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataIC50: 23nMAssay Description:In vitro inhibition of rabbit lung Angiotensin I converting enzyme (ACE) using Hippuryl-His-Leu as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of rat Angiotensin I converting enzyme (ACE), using Hip-Gly-Gly as synthetic substrate.More data for this Ligand-Target Pair
Affinity DataIC50: 156nMAssay Description:In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:In vitro inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Inhibition of Angiotensin I converting enzyme in ratMore data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:In vitro inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataIC50: 0nMAssay Description:In vitro inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+3nMAssay Description:In vitro inhibitory activity against Angiotensin I converting enzyme from unpurified guinea pig serumMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of guinea pig angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 590nMAssay Description:Inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Compound tested in vitro for inhibition of Angiotensin I converting enzymeMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Evaluation of in vitro inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Inhibitory activity against angiotensin I converting enzyme (ACE)Checked by AuthorMore data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Inhibitory concentration against angiotensin I converting enzymeMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataIC50: 3.60nMAssay Description:In vitro inhibitory activity was evaluated against angiotensin converting enzyme from rabbit in bovine buffered baseMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataIC50: 3.60nMAssay Description:In vitro inhibitory activity was evaluated against angiotensin converting enzyme from rabbit in bovine buffered baseMore data for this Ligand-Target Pair
Affinity DataIC50: 0.120nMAssay Description:Inhibition of human recombinant ACE by fluorimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of ACE by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Inhibitory activity against angiotensin converting enzyme (ACE)More data for this Ligand-Target Pair
Affinity DataIC50: 79.4nMAssay Description:Inhibition of angiotensin I converting enzyme in silicoMore data for this Ligand-Target Pair
Affinity DataIC50: 22.9nMAssay Description:Inhibitory activity against angiotensin converting enzyme (ACE)More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Inhibition of Angiotensin I converting enzyme in ratMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Ability to inhibit Angiotensin I converting enzyme was determinedMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataIC50: 23nMAssay Description:In vitro inhibitory activity against rabbit lung Angiotensin I converting enzymeMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Oryctolagus cuniculus)
Squibb Institute For Medical Research
Curated by ChEMBL
Squibb Institute For Medical Research
Curated by ChEMBL
Affinity DataIC50: 23nMAssay Description:In vitro inhibitory activity against rabbit lung Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Inhibition of Wistar rat plasma angiotensin 1-converting enzyme using H-hippuryl-His-Leu-OH as substrate after 20 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6.30nMAssay Description:Inhibition of human ACE using Hip-His-Leu-OH as substrate after 1 hr by fluorimetric methodMore data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Inhibition of ACE (unknown origin) using hippuryl-L-histidyl-L-leucine as substrate preincubated with substrate for 30 mins followed by enzyme additi...More data for this Ligand-Target Pair
Affinity DataEC50: 2.12E+4nMAssay Description:Inhibition of human ACE C domainMore data for this Ligand-Target Pair
Affinity DataEC50: 1.85E+4nMAssay Description:Inhibition of human ACE K1087A mutantMore data for this Ligand-Target Pair
Affinity DataEC50: 1.42E+6nMAssay Description:Inhibition of human ACE Y1096F mutantMore data for this Ligand-Target Pair
Affinity DataIC50: 420nMAssay Description:Inhibition of angiotensin converting enzyme (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of ACE (unknown origin)More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)